University of Oxford researchers have begun testing a COVID-19 vaccine in human volunteers in Oxford.
Around 1,110 people will take part in the trial, half receiving the vaccine and the other half (the control group) receiving a widely available meningitis vaccine.
Of the first two volunteers to take part, one likewise received the vaccine and the other the control.
The researchers started screening healthy volunteers aged between 18 and 55 in March for their upcoming trial.
Three leading members of the Oxford BRC’s Vaccines Theme are leading the trial: Prof Sarah Gilbert and Prof Adrian Hill of the University of Oxford’s Jenner Institute and Prof Andrew Pollard of the University’s Oxford Vaccine Group.
In a statement ahead of the first vaccinations, they said: “This week we will start the process of vaccine evaluation in our first human studies and are currently focussing all efforts on preparing for the start of the trials.
“Although it seems like a very long time since the work started, in reality it is less than four months since we first heard of an outbreak of severe pneumonia cases, and began to plan a response.
“Our brilliant team has been working tirelessly to get to this point using our skills and experience in vaccine development and testing, and will do the best job possible in moving quickly whilst at all times prioritising the safety of the trial participants.
The Oxford BRC has given a total of £110,000 to the vaccine trial, providing crucial funding to get the trial up and running and then helping to fund an evaluation of the safety of the vaccine.
The ChAdOx1 nCoV-19 vaccine is based on an adenovirus vaccine vector and the SARS-CoV-2 spike protein, and has been produced in Oxford.
The study aims to assess whether healthy people can be protected from COVID-19 with this new vaccine. It will also provide valuable information on safety aspects of the vaccine and its ability to generate good immune responses against the virus.
ChAdOx1 nCoV-19 is made from a virus (ChAdOx1), which is a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees. This virus has been genetically changed so that it is impossible for it to grow in humans.
Genetic material has been added to the ChAdOx1 construct that is used to make proteins from the COVID-19 virus (SARS-CoV-2) called Spike glycoprotein (S).
This protein is usually found on the surface of SARS-CoV-2 and plays an essential role in the infection pathway of the SARS-CoV-2 virus. The SARS-CoV-2 coronavirus uses its spike protein to bind to ACE2 receptors on human cells to gain entry to the cells and cause an infection.
By vaccinating with ChAdOx1 nCoV-19, the researchers are hoping to make the body recognise and develop an immune response to the Spike protein that will help stop the SARS-CoV-2 virus from entering human cells and therefore prevent infection.
Vaccines made from the ChAdOx1 virus have been given to more than 320 people to date and have been shown to be safe and well tolerated, although they can cause temporary side effects, such as a temperature, headache or sore arm.
Up to 1,102 participants will be recruited across multiple study sites in Oxford, Southampton, London and Bristol. These participants will be randomly allocated to receive either the ChAdOx1 nCoV-19 vaccine or the licensed MenACWY vaccine, which will be used as a ‘control’ for comparison.
The MenACWY vaccine is a licensed vaccine against group A, C, W and Y meningococcus that has been given routinely to teenagers in the UK since 2015 and protects against one of the most common causes of meningitis and sepsis. This vaccine is also given as a travel vaccine for high risk countries.
At the start of the trial we will also recruit a separate small group of 10 volunteers who will receive 2 doses of ChAdOx1 nCoV-19 four weeks apart.
The main focus of the study is to find out if this new vaccine is going to work against COVID-19, if it won’t cause unacceptable side effects and if it induces good immune responses. The dose used in this trial was chosen based on previous experiences with other ChAdOx1 based vaccines.
Study participants will not know whether they have received the ChAdOx1 nCoV-19 vaccine until the end of the trial.
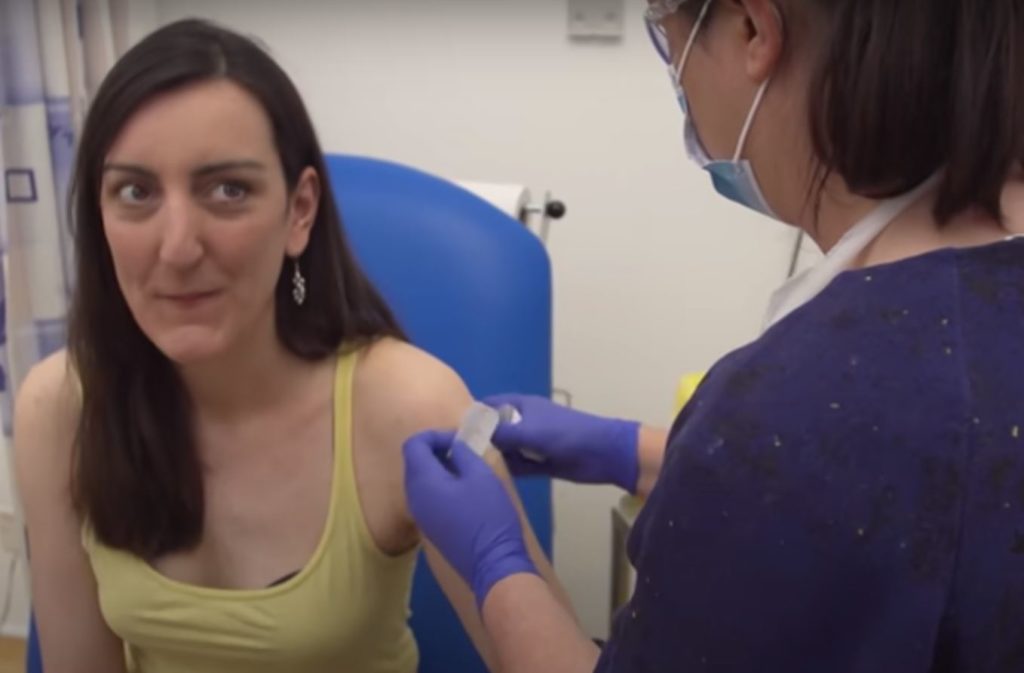
The first two participants were vaccinated on Thursday 23 April, one with the ChAdOx1 nCoV-19 vaccine and the other with the control vaccine. They will then be monitored for 48 hours.
On day three, a further six participants will be vaccinated, and then monitored for 48 hours. On nday five, the trial will progress to vaccinating larger numbers of participants.
Participants will be given an e-diary to record any symptoms experienced for seven days after receiving the vaccine. They will also record if they feel unwell for the following three weeks.
Following vaccination, participants will attend a series of follow-up visits. During these visits, the team will check participants’ observations, take a blood sample and review the competed e-diary. These blood samples will be used to assess the immune response to the vaccine.
If participants develop COVID-19 symptoms during the study, they can contact a member of the clinical team, who will assess whether they have become infected with the virus. If a participant was very unwell, we would call our colleagues in the hospital and ask them to review the volunteer if appropriate.
To assess whether the vaccine works to protect from COVID-19, statisticians in the research team will compare the number of infections in the control group with the number of infections in the vaccinated group. For this purpose, it is necessary for a small number of study participants to develop COVID-19.
How quickly we reach the numbers required will depend on the levels of virus transmission in the community. If transmission remains high, we may get enough data in a couple of months to see if the vaccine works, but if transmission levels drop, this could take up to six months.