Gene and cell therapies are being trialled for a number of sight-loss conditions, with Oxford playing a leading role.
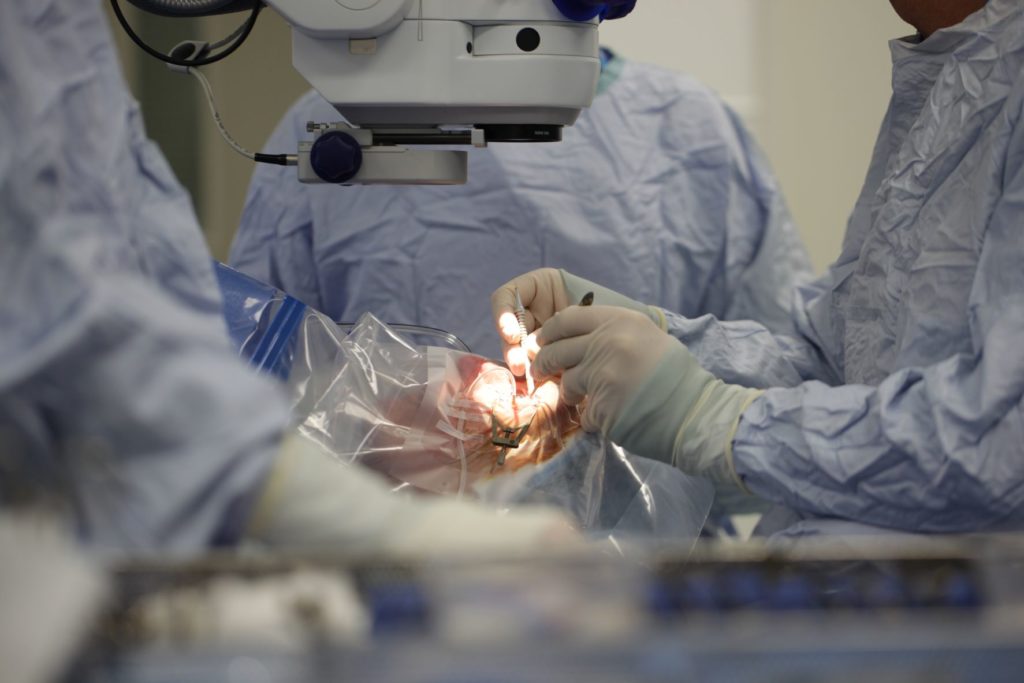
With Oxford BRC support, a clinical trial has recently been conducted to evaluate transplantation of photoreceptor cells derived from stem cells to treat retinitis pigmentosa (RP), the commonest cause of blindness in the young.
The first-in-human clinical trial at Oxford of a gene therapy for age-related macular degeneration (AMD), the commonest cause of vision loss in older people, has been expanded to many international sites, including Australia, and is about to go into a Phase III clinical trial led by Novartis.
In addition, the Oxford Eye Hospital is one of only three centres in the UK chosen to administer voretigene neparvovec (Luxturna™), a gene therapy made available by the NHS for treatment of Leber congenital amaurosis, a rare condition that starts in early life and causes vision to deteriorate progressively towards complete blindness. Luxturna is the first ever gene therapy approved by the NHS for patient treatment, which demonstrates the Oxford BRC’s commitment to translate research in gene therapy into treatments.
Age-related macular degeneration (AMD)
In a clinical trial sponsored by Gyroscope Therapeutics, a UK-based company developing genetically defined therapies for the treatment of eye diseases, the Oxford BRC-supported team carried out the world’s first gene therapy operation to tackle the cause of AMD, which affects more than 600,000 people in the UK.
Dry AMD is caused by a slow deterioration of cells in the macula. It affects the central part of a patient’s vision with gaps or ‘smudges’, making everyday activities like reading and recognising faces difficult.
The operation involves detaching the retina and injecting a solution containing a virus underneath. The virus contains a modified DNA sequence, which infects cells, called the retinal pigment epithelium (RPE), and corrects a genetic defect that causes AMD.
The aim of the therapy is to halt the progress of the condition, preserve the remaining sight, and improve their quality of life. If successful, it is hoped that gene therapy can be used in the future on patients with early AMD and halt the disease before their vision has started to deteriorate significantly.
We are now investigating whether we can improve this treatment, for example by developing a single gene therapy treatment or improving immune suppression to reduce side effects.
Cell therapy for retinitis pigmentosa
Oxford University Hospitals was the first UK centre to carry out a revolutionary new treatment where stem cells are transplanted into the retina to replace lost photoreceptors as a way of treating retinitis pigmentosa (RP) – an inherited degenerative cause of blindness.
The photoreceptor transplantation procedure was performed as part of a phase 2a clinical trial led by the UK-based stem cell therapeutics company ReNeuron. It involves stem cell-derived progenitor cells being transplanted into the retina, with a view to them becoming photoreceptors.
RP is a group of inherited retinal disorders where the photoreceptors in the retina progressively deteriorate, leading to tunnel vision and sometimes a complete loss of vision. It is the most common cause of blindness in the working population in the UK, affecting approximately one in 4,000 people.