Researchers in Oxford have carried out the world’s first gene therapy operation to tackle the root cause of age-related macular degeneration (AMD), the UK’s most common cause of sight loss.
(Jon Brett, Eye Research Group Oxford)
The procedure was carried out at the John Radcliffe Hospital by Prof Robert MacLaren, Professor of Ophthalmology at the University of Oxford, with the support of the NIHR Oxford Biomedical Research Centre in a clinical trial sponsored by Gyroscope Therapeutics, a UK-based company developing genetically-defined therapies for the treatment of eye diseases.
Prof MacLaren says: “AMD is the number one cause of untreatable blindness in the developed world. A genetic treatment administered early on to preserve the vision in patients who would otherwise lose their sight would be a tremendous breakthrough and certainly something I hope to see in the near future.”
Age-related macular degeneration (AMD) is the biggest cause of sight loss in the UK, affecting over 600,000 people. Dry AMD is a slow deterioration of the cells of the macula. It affects the central part of a patient’s vision with gaps or ‘smudges’, making everyday activities like reading and recognising faces difficult.
If successful, the treatment could have a beneficial impact of patients’ quality of life and their ability to remain independent.
The first person to undergo the procedure was Mrs Janet Osborne of Oxford. Like many people with AMD, she has the condition in both eyes, but it is more advanced in her left eye. As is typical with this condition, the central vision in her left eye has deteriorated and is very hazy, although her peripheral vision is better.
The 80-year-old says that her restricted vision makes household tasks like preparing vegetables and sewing difficult, and she cannot read for very long. Often she finds it hard to recognise faces.
She says her motivation for taking part in the trial was the possibility of helping others with AMD: “I wasn’t thinking of me. I was thinking of other people. For me, I hope my sight doesn’t get any worse. That would be fantastic. It means I wouldn’t be such a nuisance to my family.”
The operation was part of the FOCUS trial, sponsored by Gyroscope Therapeutics, a UK biotech company developing gene therapy products for ocular diseases such as dry AMD, which was founded and built by Syncona. Some of the enabling viral vector development took place at the University of Oxford with NIHR funding.
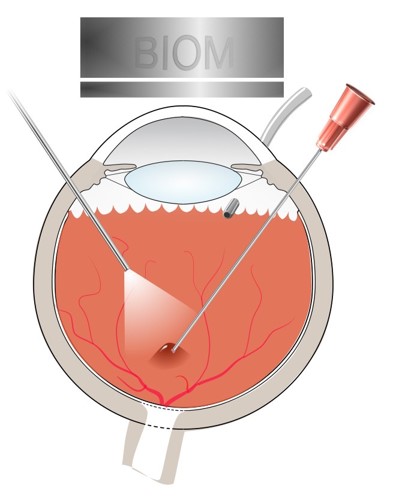
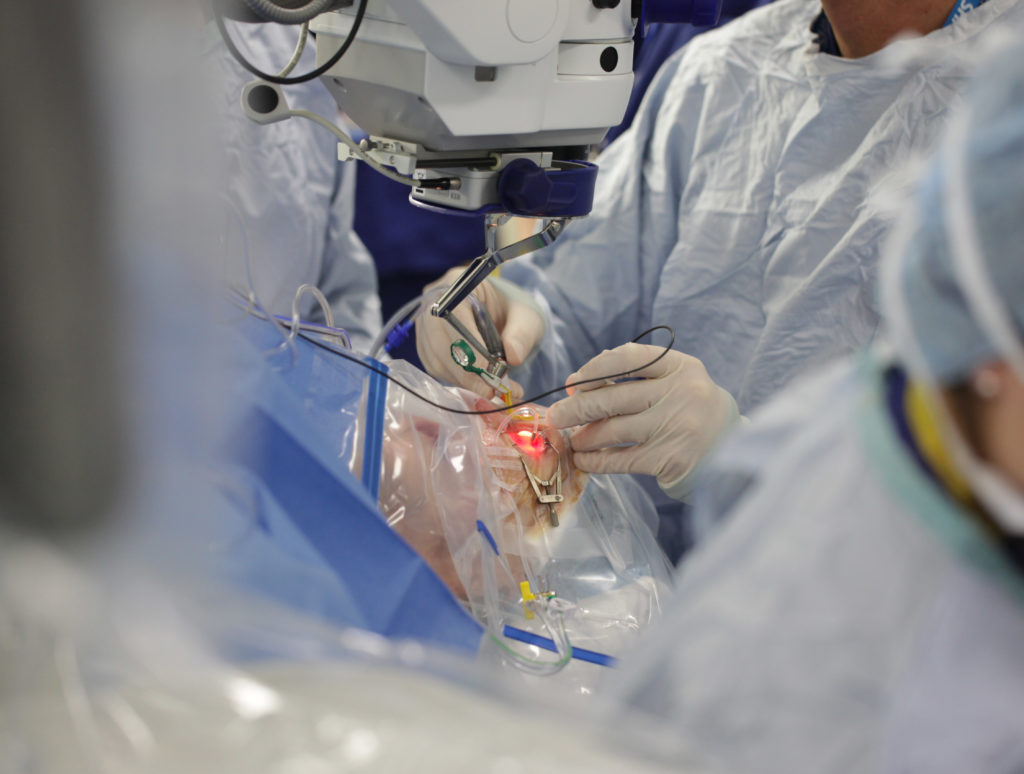
The operation involves detaching the retina and injecting a solution containing a virus underneath. The virus contains a modified DNA sequence, which infects cells, called the retinal pigment epithelium (RPE), and corrects a genetic defect that causes AMD. Ideally if successful, gene therapy would only need to be performed once, as the effects are thought to be long-lasting.
A key factor in AMD is the complement system, a system of proteins in our immune system that fights bacteria. In macular degeneration, these proteins are over-active and start to attack the retinal cells, in a similar way to how they would attack bacteria.
Prof MacLaren explains: “We’re harnessing the power of the virus, a naturally occurring organism, to deliver the DNA into the patient’s cells. When the virus opens up inside the retinal cell it releases the DNA of the gene we have cloned, and the cell starts making a protein that we think can modify the disease, correcting the imbalance of the inflammation caused by the complement system.
“The idea of this gene therapy is to ‘deactivate’ the complement system, but at a very specific point at the back of the eye, so the patient would otherwise be unaffected by it, and we hope that in future it will slow down the progression of macular degeneration.”
Sir Peter Lachman, the scientist from the University of Cambridge who led the pioneering work on the complement system leading to the creation of Gyroscope Therapeutics, said: “We have a better understanding now on the relationship between the complement system and the AMD disease which lead us to the discovery that restoring the balance of a hyperactive complement system could be a potential therapeutic approach in dry AMD”.
The aim of the therapy is to halt the progress of the condition and preserve what vision patients have remaining. If successful, it is hoped that gene therapy can be used in the future on patients with early AMD and so halt the disease before their vision has started to deteriorate.
Prof MacLaren ran the first gene therapy clinical trials from Oxford for rarer causes of blindness, Choroideremia and retinitis pigmentosa.
Prof MacLaren said: “This is a rapidly evolving field. Given that we understand a lot more now about the manufacture of the treatment, and the effects of the virus when doing gene therapy at the back of the eye, as well as all the other gene therapy programmes being developed at the moment, I would hope that we’ll see a treatment for people with dry AMD within the next few years,” he said.