Led by Professor Simon Draper
The ‘big three’ account for around 2.4 million deaths worldwide every year. This figure is expected to rise following the COVID-19 pandemic, with growing tuberculosis drug resistance and co-infection with HIV, further driving pressure on the NHS.
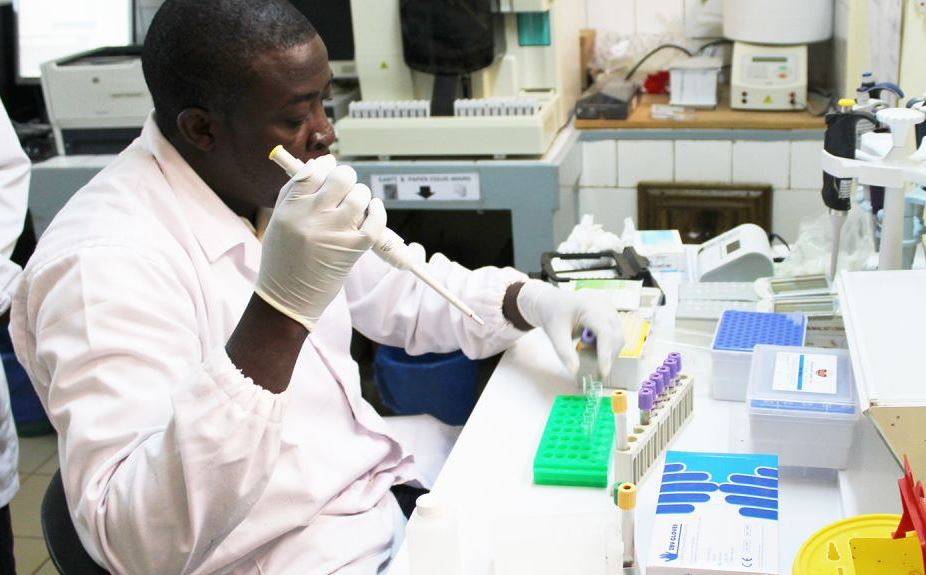
The current BRC R21/Matrix-M anti-infection plasmodium falciparum malaria vaccine was licenced to the Serum Institute of India, having been shown to be 77 percent effective. Phase 3 trials of this vaccine are under way.
We are also testing next-generation plasmodium falciparum vaccines using multi-stage and structure-based design. Vaccines against plasmodium vivax, which is common in travellers returning to the UK, will be tested in a human infection trial. Read more about our work on malaria vaccines.
As well as testing next-generation TB vaccines, we are also exploring new ways of delivering those vaccines effectively. This includes trialling aerosol to deliver TB vaccines, and then undertaking a human infection challenge model to select the most promising candidate vaccines.
As part of the NIHR CHERUB collaboration, which is looking for a cure for HIV, we are supporting latency-targeting vaccines and other immunotherapeutic approaches.