A new study has found that a drug recently licensed in the UK has no effect on post-operative knee replacement recovery or pain, compared to the current treatment when administered at the site of surgery.
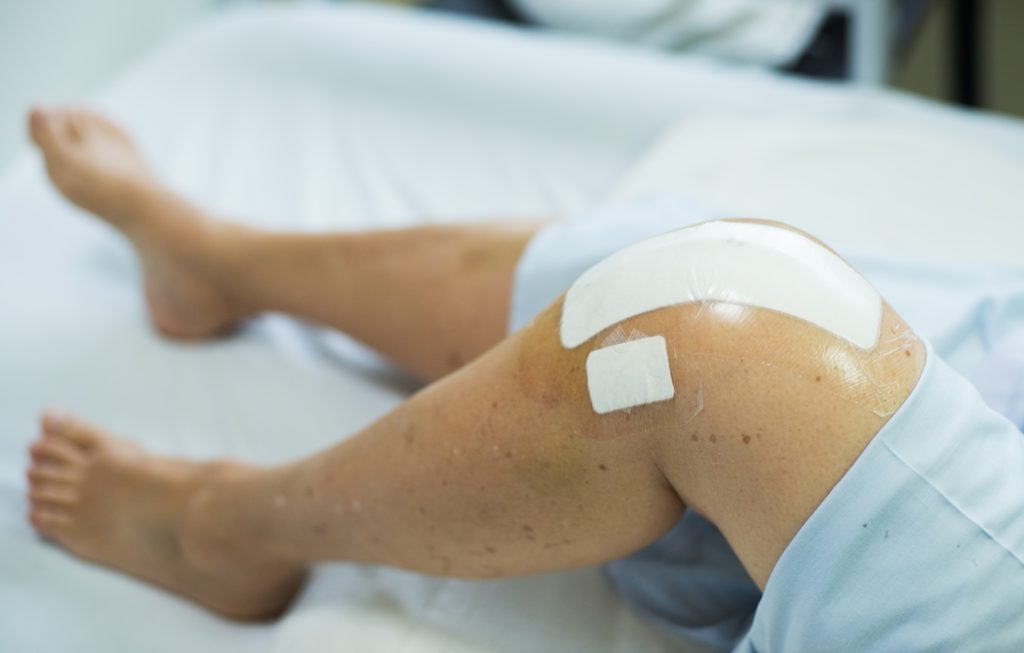
Knee replacement surgery is highly successful for treating severe arthritis; 100,000 people undergo the procedure every year in the UK, with numbers set to rise significantly in future.
However, nearly half of patients report severe post-operative pain. Currently pain control is provided by injecting a local anaesthetic of bupivacaine hydrochloride around the knee during surgery providing good pain relief for 12 to 24 hours. However, patients typically experienced the worst pain the next morning when they are encouraged to bend their knee and get out of bed.
Researchers at the Universities of Oxford and Leeds developed the SPAARK (Study of Peri-Articular Anaesthetic for Replacement of the Knee) Trial, to test whether liposomal bupivacaine, a post-operative pain treatment widely used in the USA would be more effective at managing the pain compared to current treatments. The findings have been published in JAMA.
The trial involved the University of Oxford’s Surgical Intervention Trials Unit (SITU), which is supported by the NIHR Oxford Biomedical Research Centre. The research was funded by a grant from the NIHR Research for Patient Benefit Programme.
Lead author Thomas Hamilton, Academic Clinical Lecturer in Trauma and Orthopaedic Surgery at the University’s Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS), said: “We found that liposomal bupivacaine injected at the surgical side during knee replacement did not improve post-operative recovery, compared to those receiving bupivacaine hydrochloride alone.
Prof Hemant Pandit (Professor of Orthopaedic Surgery, University of Leeds and University of Oxford), senior author and chief investigator of the study, added: “This is the largest randomised controlled trial in the world to assess the effectiveness of liposomal bupivacaine in achieving superior pain relief in patients undergoing a knee replacement.
“The study results are timely and demonstrate that there is no additional benefit in the pain relief experienced by patients receiving Liposomal Bupivacaine and therefore the drug’s use in routine NHS practice cannot be justified.”