The first participants in a clinical trial of a bioelectrical therapy to treat incontinence have received their ‘smart’ bioelectronic implants.
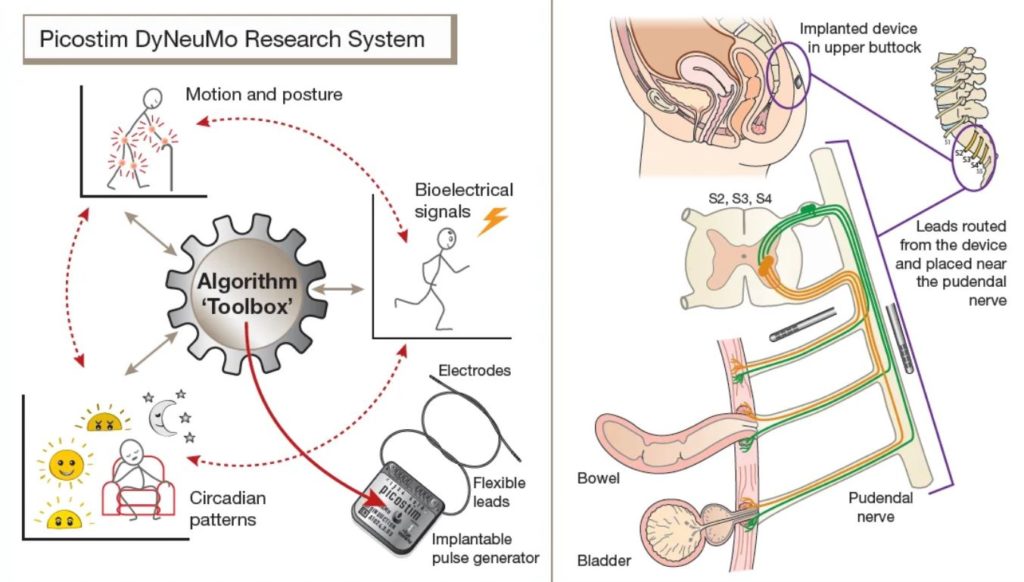
Amber Therapeutics, a University of Oxford spin-out company developing intelligent bioelectrical therapies, applied the Picostim-DyNeuMo research system developed in collaboration with Bioinduction Ltd. The AURA-2 study represents the latest use of the Picostim-DyNeuMo research platform, which was first implanted at Oxford last year for the treatment of Parkinson’s-like multiple system atrophy.
The Picostim-DyNeuMo research system toolbox was developed as a general-purpose bioelectronic therapy development platform. For the AURA-2 study, leads are placed along the pudendal nerve and the implanted system provides stimulation patterns under the control of the research participant.
This research is supported by the NIHR Oxford Biomedical Research Centre (BRC). This kind of neuromodulation is an important area of research in the BRC’s new Surgical Innovation, Technology and Evaluation Theme.
Urinary incontinence
Urinary incontinence is a debilitating medical condition that affects 8.5 percent of the global population. It can bring about a sense of loss of control and shame, leading to isolation and depression.
Charles Knowles, Chief Medical Officer of Amber TX and Professor of Surgery at Queen Mary University of London, states: “Urinary incontinence is one of the most common medical problems in humans. It causes misery for millions of men and women worldwide. Amber’s therapy represents the first advanced therapy directed to mixed urinary incontinence. Our study, to determine if this new treatment is safe, will be a major step forward in a field that has seen little real innovation for over a quarter of a century.”
Closed-loop neuromodulation
Amber Therapeutics uses its expertise in ‘closed-loop neuromodulation’ to transform clinical outcomes in patients with functional disorders of the peripheral nervous system. Closed-loop neuromodulation is a type of therapy that adjusts its stimulation parameters according to measurements of the nervous system’s needs in real time.
In the AURA-2 study, investigators are exploring how to directly regulate the urge to empty the bladder (‘urge incontinence’) while also increasing resistance to urine leakage caused by activities such as coughing or lifting (mixed urinary incontinence’).
The implanted device can sense, interpret, adapt and respond to individual patient commands in an attempt to restore normal bladder function. The device is inserted in participants’ pelvic region using a minimally invasive surgical procedure that accesses and targets the pudendal nerve – the nerve that directly controls continence.
Professor Tim Denison is Chief Scientific Officer of Amber Therapeutics and RAEng Chair in Emerging Technology at the Department of Engineering Science and Nuffield Department of Clinical Neurosciences at the University of Oxford. His research is supported by the Oxford BRC.
He said: “Modern bioelectronic systems have the unique capability to measure physiological signals and adjust stimulation in real-time. In partnership with clinicians, we can create novel adaptive, reflex-like, algorithms for exploring new therapies.
“The flexibility of these systems allows us to use software updates to configure to different disease states. The AURA-2 study builds on the prior trial in Multiple System Atrophy, and paves the way for emerging therapies in generalised epilepsy and chronic pain. We are grateful for our partnership and collaboration with Bioinduction, and their support of the state-of-the-art Picostim-DyNeuMo research system, which has allowed us to rapidly explore our first UI therapy concept.”
The clinical trial
To date, five participants have been safely implanted with the Amber-UI system with the adaptive algorithm activated and running continuously in a home setting. The remaining participants will be enrolled during the first half of 2023.
Early indications confirming the feasibility of the surgical procedure and therapy are very promising. The study is expected to conclude towards the end of 2023, with learnings used to improve and optimise the Amber-UI therapy in preparation for a large-scale trial.