A randomised controlled trial comparing extracapsular hip fracture fixation devices, conducted by Oxford University and supported by NIHR Oxford Biomedical Research Centre, has reached a key milestone, with the 1000th patient recruited completing their one-year follow-up
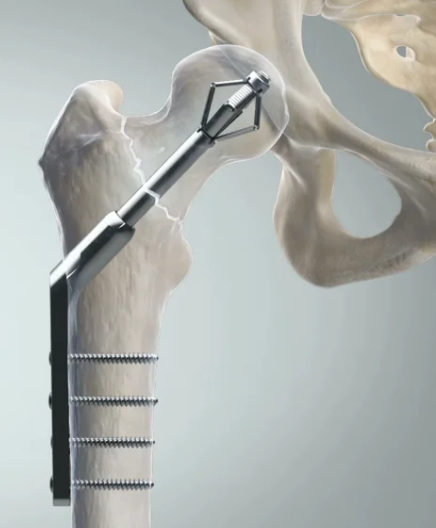
Recruitment for the World Hip Trauma Evaluation 4 (WHITE4) began in June 2016 and involved 10 centres in the UK, in collaboration with the Orthopaedic Trauma Society, and the Irish company X-Bolt Orthopaedics, whose innovative hip fracture device is being used in the trial.
Addressing the growing socio-economic problems of treating hip fractures in frail elderly patients, X-BOLT is designed to revolutionise current surgical treatment and significantly reduce re-operation rates.
Most clinical complications occur with anchorage in the osteoporotic bone and the device offers an innovative mechanism that uses wings that expand in situ to offer a rotationally stronger and more secure femoral bone anchorage than traditional screw fixation.
A stronger leg to stand on post-surgery allows for faster, fuller and more confident mobilisation of the patient.
Patients recruited to the trial were randomised at the time of surgery to receive either a gold standard sliding hip screw (SHS) device or X-BOLT. Follow-up for all patients occurred at baseline, four months and one year after surgery.
A 100-patient randomised pilot study published in 2016, known as ‘WHITE1’ at the University of Warwick showed a zero (0%) re-operation rate with the X-BOLT device, versus a 6% reoperation rate with the traditional SHS. in unstable intertrochanteric hip fractures.
Professor Xavier Griffin, the Chief Investigator of the WHITE 4 trial at Oxford Trauma, commented on the trial, “This is the largest randomised clinical trial in the field of hip fracture fixation and we are delighted to have hit this significant milestone. X-Bolt should be congratulated as an exemplar company for their commitment to working with us using the IDEAL framework in delivering this novel device to market. We look forward to communicating the full results of the trial in June 2019.”
Dr. Brian Thornes, CEO of X-BOLT Orthopaedics said, “Hip fractures in the very elderly are a growing unmet medical need with current surgical procedures resulting in poor outcomes that often result in a loss of mobility and independence in vulnerable group of patients. Hip fracture fixation has lacked any significant innovation since Sir John Charnley patented the sliding hip screw in 1955. We believe that the X-BOLT® device has the potential to transform fixation in patients suffering from osteoporosis and hip fractures, providing a significant improvement in mobility and quality of life.”
Approximately 1.6 million hip fractures occur worldwide each year and, due to an aging population, it is estimated this number could reach 6 million by 2050. Each hip fracture episode costs approximately £30,000 in health and social costs. Loss of mobility and independence among hip fracture survivors is profound; less than 50% regain their previous function and 33% are totally dependent or in a nursing home a year later.
Reducing the re-operation rate provides a significant opportunity for hospitals and governments to reduce 30-day readmissions and overall healthcare costs, notwithstanding the benefits to patients by improving their quality of life.